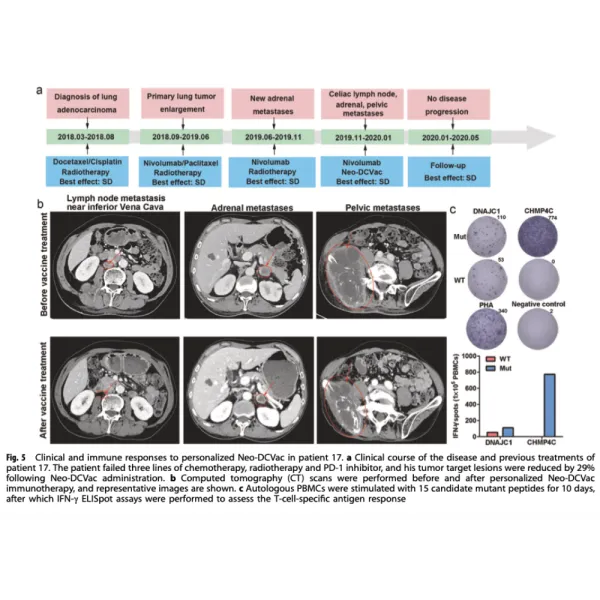
In this groundbreaking study, lung cancer patients are treated with a personalized neoantigen peptide-pulsed autologous DC vaccine, representing a promising advancement in the field of lung cancer treatment. The approach of targeting neoantigens has shown great potential in activating the body’s immune response against cancer cells. The personalized nature of the vaccine allows for tailored treatment plans that take into account individual patient characteristics, ultimately leading to better outcomes and quality of life.
Patients with histologically or cytologically confirmed lung cancer, including squamous cell carcinoma, adenocarcinoma, neuroendocrine tumor, and SCLC, who were aged between 18 and 75 years with at least one measurable disease according to the RECIST. Patient must have undergone standard of care therapies and have progressed diseases. The life expectance should be at least 3 months. Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, as well as adequate bone marrow, renal, and hepatic functions.
DNA and RNA preparation
Tumor tissues were obtained by fiber bronchoscopy biopsy, supraclavicular metastatic lymph node resection, fine needle aspiration of metastatic lumbar lesion and fine needle aspiration of lung tumor lesions and stored with 1 ml of RNA later (Thermo Fisher, #AM7020). Blood was collected and mixed with an EDTA- based anticoagulant. DNA and RNA were extracted from the same tumor tissue with the All Prep DNA/RNA Mini Kit, and DNA from EDTA-anti coagulated peripheral blood was extracted with the QIAamp DNA Blood Mini Kit. Quality of DNA/ RNA was evaluated by 2100 Bioanalyzer. Trio samples (two DNA samples from tumor and blood, and one RNA sample from only the tumor) for each patient were prepared for the subsequent sequencing process.
DNA and RNA preparation
Tumor tissues were obtained by fiber bronchoscopy biopsy, supraclavicular metastatic lymph node resection, fine needle aspiration of metastatic lumbar lesion and fine needle aspiration of lung tumor lesions and stored with 1 ml of RNA later (Thermo Fisher, #AM7020). Blood was collected and mixed with an EDTA- based anticoagulant. DNA and RNA were extracted from the same tumor tissue with the All Prep DNA/RNA Mini Kit, and DNA from EDTA-anti coagulated peripheral blood was extracted with the QIAamp DNA Blood Mini Kit. Quality of DNA/ RNA was evaluated by 2100 Bioanalyzer. Trio samples (two DNA samples from tumor and blood, and one RNA sample from only the tumor) for each patient were prepared for the subsequent sequencing process.
PBMCs were isolated from patients’ peripheral blood. 5–10 × 106/ml elutriated PBMCs were inoculated into T175 flasks containing AIM-V medium and incubated for 3 hat 37 °C and 5% CO2. Then, the suspended cells were collected and frozen in liquid nitrogen. The adherent cells were washed and cultured in AIM-V medium containing 1% autologous serum, clinical grade human GM-CSF (1000 IU/ml) and animal-free research grade IL-4 (500 IU/ml). After 5 days, the neoantigen peptides (50 μg/ml) were added to the immature DCs. Following 20–24 hours peptide loading, DCs were matured with TNF-α (10 ng/ml), IL-1β (10 ng/m), IFN-γ (1000 U/ml), prostaglandin E2 (PGE2, 250ng/ml) R848 (1μg/ml), and polyinosinic- polycytidylic acid (20 ng/ml; poly (I:C)) and incubated for 20–24 hours.
Patients were pretreated with cyclophosphamide at a dose of 250 mg/m2 1 day before injection of Neo- DCVac. The prepared Neo-DCVac was vaccinated subcutaneously at both axillary and inguen regions bilaterally at day 0. GM-CSF was administered at a dose of 0.075 mg during the following 5 days (days 1– 5).
AEs and Toxicity
All adverse events were limited to grade 1 or 2. All patients experienced grade 1 skin injection-site reactions. Patient 4 developed transient grade 1 neutropenia after receiving Neo-DCVac treatment, which was relieved without any treatment. Patient 12 developed a grade 2 itchy rash throughout his trunk and extremities. No other specific treatment was administered, and his rash waned after vaccination ceased. Neo-DCVac did not increase the risk of immune-related adverse events related to ICIs. No toxicity was dose-limiting or resulted in dose delay or treatment discontinuation.
The median progression-free survival (PFS) was 5.5 months (95% confidence interval (CI, 1.9–9.2), and the median overall survival (OS) was 7.9 months (95% CI, 5.9–10.0). Three (25%) of 12 patients achieved an objective response. All of the responses were PR, with no complete responses (CRs) recorded. Six (50%) of 12 patients experienced a decrease in the size of their target lesions, nine (75%) of 12 patients achieved disease control, and progressive disease (PD) was recorded in three patients (25%).
This findings from this pilot study have proven that Neo- DCVac is feasible, safe, and capable of eliciting specific T-cell immunity and therapeutic benefit. The first findings for the activity of a neoantigen-based DC vaccine treatment in patients with advanced NSCLC. Perhaps of greatest importance, Neo-DCVac could have implications in a wide range of cancers.
Article Reference link: click here
Scientific article publishing date 1/21/2021
REGISTERED ADDRESS:
Immunyo Ltd
Kincora Ave 35
Clontarf
Dublin 3
Ireland
For Germany, Austria and Switzerland