In this study, the safety and survival outcomes of melanoma patients with regional metastatic disease who underwent a Regional Lymph Node Dissection (RLND) and received adjuvant Dendritic Cell (DC) vaccination are analyzed. By assessing the safety and efficacy of this treatment approach, the hope is to provide new therapeutic options for melanoma patients facing regional metastatic disease. The use of DC vaccination as an adjuvant therapy after RLND has shown promise in preclinical studies and may help to enhance anti-tumor immunity in these patients.
The incidence of cutaneous melanoma continues to rise worldwide. Adequate surgical resection remains the standard of care for patients with non-systemic disease. However, approximately 15–20% of patients with cutaneous melanoma will develop regional (lymph node) metastasis. DCs, the most effective antigen-presenting cells of the immune system, are exploited to induce melanoma-specific cytotoxic T cells in melanoma patients. Immature DCs are very effective in antigen uptake and when stimulated by inflammatory mediators and ‘danger signals’ they mature and migrate from peripheral tissues to lymphoid organs.
Patients are divided into two groups.
Immunotherapy group (n=78) patients with melanoma received adjuvant Dendritic cell vaccine.
DC vaccination consisted of a maximum of three cycles of three biweekly vaccinations. Of all the patients, 54 of 78 patients received the complete three cycle of vaccination.
Dendritic cell vaccination
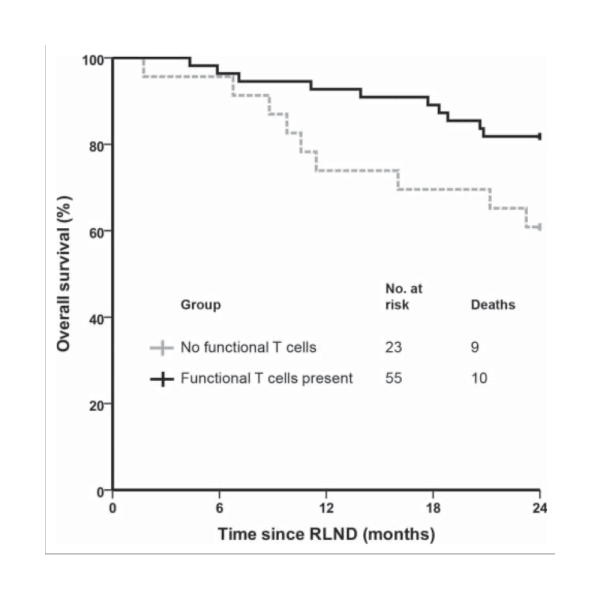
Functional tumor-specific T cells were detected in 55 out of 78 vaccinated patients (71%).The 2-year survival rate was significantly higher (82%) in patients with functional tumor-specific T cells compared to DC vaccinated patients without functional tumor-specific T cells 61% (p = 0.04).
Toxicity
DC vaccinations were generally well tolerated. Forty-eight out of 57 patients (84%) receiving only DC vaccinations and 20 out of 21 patients (95%) receiving both DC vaccination and IL-2 suffered at least one mild adverse event (CTC grade 1 or 2)
The most common side effects that are associated with DC vaccination are transient flu-like symptoms, including fatigue and fever and erythema at the site of injection. No treatment related grade 3 or 4 toxicity was observed. Treatment was discontinued in two patients at their own request due to vaccine-related grade two rash in the second cycle, both patients are still alive (94 and 115 months after RLND).
Survival
The median OS increased more than two-fold in patients who received adjuvant DC vaccination as compared with the control group, from 31.0 (95% CI 23.6–38.5) to 63.6 months (95% CI 24.5– 102.7) (p = 0.018). For patients received DC vaccination (n=78), the 1-year OS rate is 87%, 2-year OS rate is 76% and 5-year survival rates is 53% And for Control group (n=209), the 1-year OS rate is 74%, 2-year OS rate is 59% and the 5-year OS rate is 38% (p = 0.018; p = 0.009; p = 0.008, respectively). For the time to distant metastasis a trend was seen in favor of the DC vaccinated patients, with a median of 41.9 months (95% CI 32.3–51.4) vs. 24.3 months in the control group (95% CI 18.9– 29.7; p = 0.081)
Adjuvant treatment with DC vaccination after RLND in patients with regional metastasis of melanoma is safe and results in a favorable OS compared to matched controls. Importantly, DC
vaccination is well tolerated and clearly less toxic than adjuvant IFN-α or ipilimumab. These results suggest that DC vaccination has efficacy as adjuvant treatment of melanoma and provide further support to test this in a prospective randomized clinical trial.
REGISTERED ADDRESS:
Immunyo Ltd
Kincora Ave 35
Clontarf
Dublin 3
Ireland
International
For Germany, Austria and Switzerland