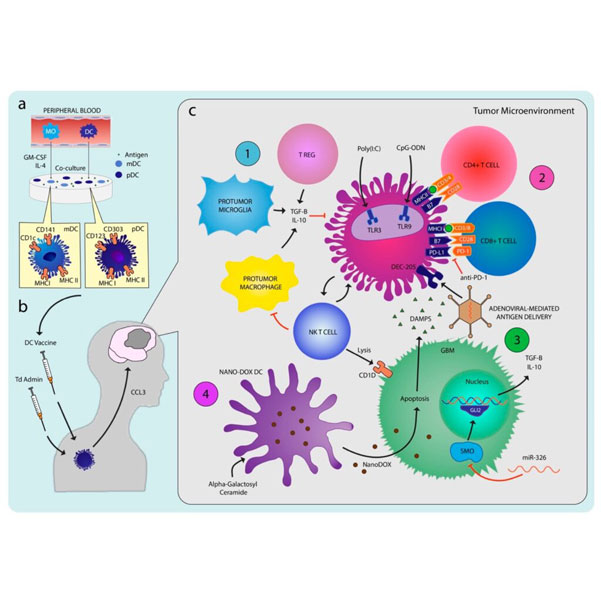
Glioblastoma (GBM) is a devastating primary central nervous system malignancy in adults, with limited treatment options and an average survival rate of less than 15 months. While surgery, radiation, and chemotherapy are the standard treatments, they only offer modest benefits and recurrence is common. Given the poor prognosis of GBM, there is an urgent need for novel therapies. Immunotherapy utilizing dendritic cells (DCs) has emerged as a promising approach to enhance the body’s anti-tumor immune response. As “professional” antigen-presenting cells, DCs play a crucial role in initiating immune responses against tumors. Preclinical studies have demonstrated prolonged survival and immune memory in murine GBM models with stimulation of DC activity using various antigens and costimulatory molecules.
De Vleesvchouwer et al. and Wheeler et al. conducted large phase II trials with 56 GBM and 23 GBM patients, respectively. In De Vleesvchouwer’s study, patients >3 years of age were enrolled into a prospective cohort comparison trial (HGG-IMMUNO) in which recurrent GBM patients were treated with DCVs pulsed with autologous tumor cells in three cohorts, where each cohort served as historical control for the next cohorts. In cohort A, DCV was given at week 1 and 3 and then every 4 weeks. In cohort B, five DC vaccinations were given at 2-week intervals and then every 4 weeks. In cohort C, 4 weekly DC vaccinations were given with boosters of intradermal injections of tumor lysate. The authors found a trend for improved PFS and OS in patients younger than age 35. A subgroup analysis of patients greater than age 21 showed an improved PFS and OS in cohort C, patients treated with weekly vaccination administrations and boosters. Wheeler et al. studied the efficacy of tumor lysate pulsed DCV administered subcutaneously in 33 GBM patients, 23 recurrent and 11 newly diagnosed, at 2-week intervals for three doses and a fourth vaccination 6 weeks after the third. One patient developed metastatic GBM around the site of the vaccine injection and was thought to be due to the growth of rare radiation-resistant tumor cells present in this particular patient and not from metastasis from the original primary tumor. PFS and OS in vaccinated individuals compared favorably with patients who did not undergo DCV treatment at the trial institution during the time of the trial. Seventeen out of 34 GBM patients exhibited a positive vaccine response with seven exhibiting >1.5-fold increase in IFN-γ production before vaccination, which is suggestive of endogenous anti-tumor response. They found that the median survival in vaccine responders was 642 days compared to 430 days in vaccine non-responders. PFS was 308 days in vaccine responders compared to 167 days in vaccine non-responders.
Preclinical and clinical studies have demonstrated measurable immunological response and variably prolonged survival rates. Various combinations of synergistic adjuvants aimed at overcoming the diverse glioma-induced immunosuppression have shown promise. The interim report from the first Phase III trial of DCV in newly diagnosed GBM confirms safety and feasibility and suggests longer than expected survival with DCV. While we eagerly await the final results from the study and results from other Phase III studies, there is need to further explore the optimal combination of immune-based therapies, ideal integration of these therapies into the current standard of care, and responder phenotypes to identify patients who are most likely to benefit from the therapy.
REGISTERED ADDRESS:
Immunyo Ltd
Kincora Ave 35
Clontarf
Dublin 3
Ireland
International
For Germany, Austria and Switzerland